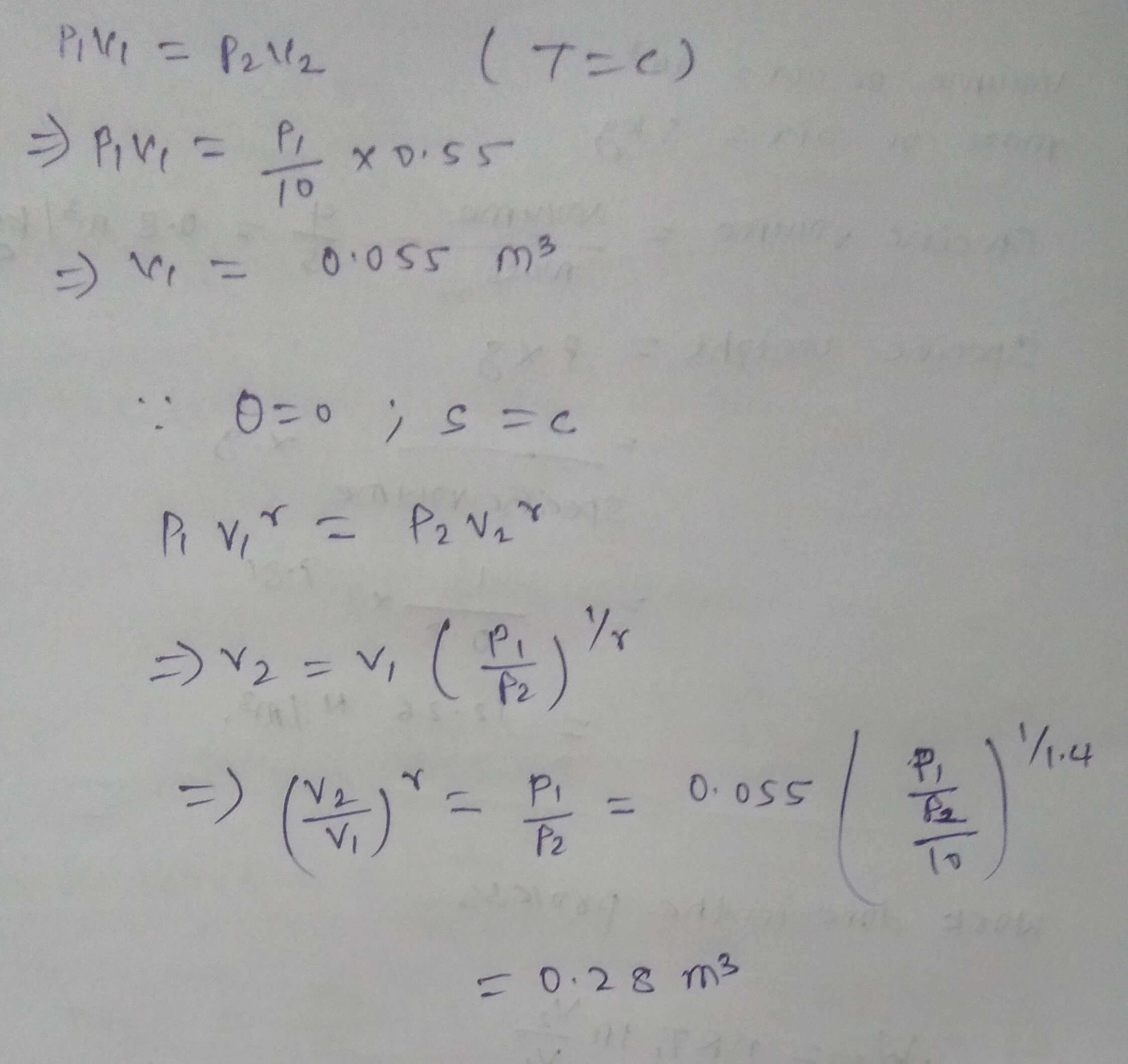A gas expands from pressure P1 to pressure P2(P2=P1/10). If the process of expansion is isothermal, the volume at the end of expansion is 0.55 m^3. If the process of expansion is adiabatic, what is the volume at the end of expansion?
Answered
A gas expands from pressure P1 to pressure P2(P2=P1/10). If the process of expansion is isothermal, the volume at the end of expansion is 0.55 m^3. If the process of expansion is adiabatic, what is the volume at the end of expansion?