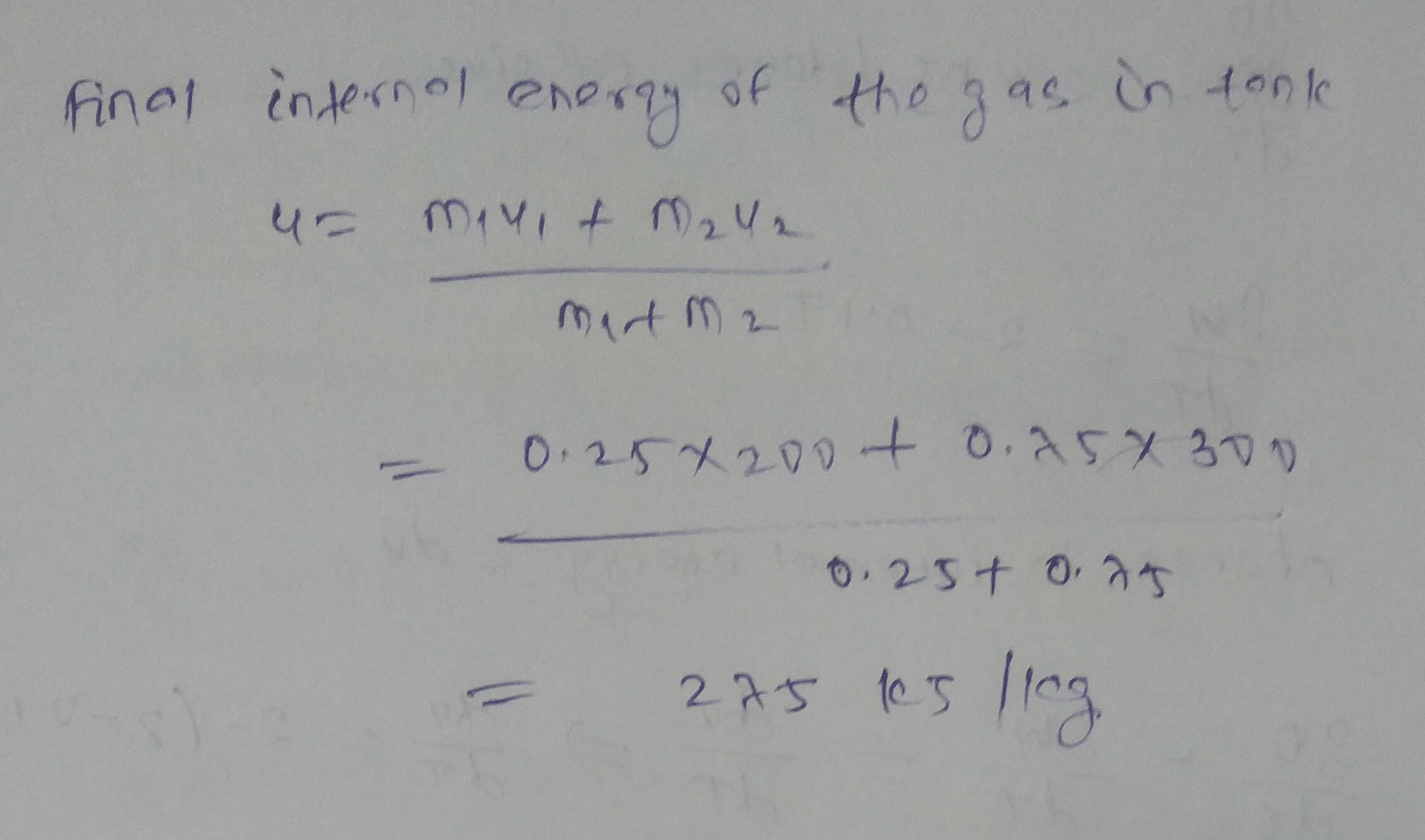An insulated tank initially contains 0.25 kg of gas with an internal energy of 200 kJ/kg. Additional gas with an internal energy of 300 kJ/kg and an enthalpy of 400 kJ/kg enters the tank until the total mass of gas contained is 1 kg. What is the final internal energy (in kJ/kg) of the gas in the tank?
Answered
An insulated tank initially contains 0.25 kg of gas with an internal energy of 200 kJ/kg. Additional gas with an internal energy of 300 kJ/kg and an enthalpy of 400 kJ/kg enters the tank until the total mass of gas contained is 1 kg. What is the final internal energy (in kJ/kg) of the gas in the tank?